


Adverse Event reporting information can be found in footer
Request a Meeting

*Scottish Medicines Consortium (SMC) advice: desmopressin oral lyophilisate (Noqdirna®) is accepted for restricted use within NHS Scotland. Indication under review: Symptomatic treatment of nocturia due to idiopathic nocturnal polyuria in adults. SMC restriction: For use in patients aged 65 years and over. Two Phase Ill, placebo-controlled studies demonstrated that desmopressin, at licensed doses over three months, significantly reduced the mean number of nocturnal voids and resulted in higher proportions of responders compared to placebo, in patients with nocturia.5
*All Wales Medicines Strategy Group (AWMSG) advice: Desmopressin acetate (Noqdirna®) for the treatment of nocturia due to idiopathic nocturnal polyuria in adults is recommended for restricted use within NHS Wales. Desmopressin acetate (Noqdirna®) should be restricted for use in the following subpopulation within its licensed indication for the treatment of nocturia due to idiopathic nocturnal polyuria in adults: aged over 65, for whom treatment options are currently limited. Desmopressin acetate (Noqdirna®) is not recommended for use within NHS Wales outside of this subpopulation.6
Patients over 65 should be monitored for sodium at treatment initiation, 4-8 days after treatment initiation and at one month to reduce risk of hyponatraemia. Noqdirna should be discontinued if the serum sodium level falls below the lower limit of the normal range (ie 135 mmol/L).1
†The Noqdirna Summary of Product Characteristics (SmPC) does not recommend co-administration with other therapies.1
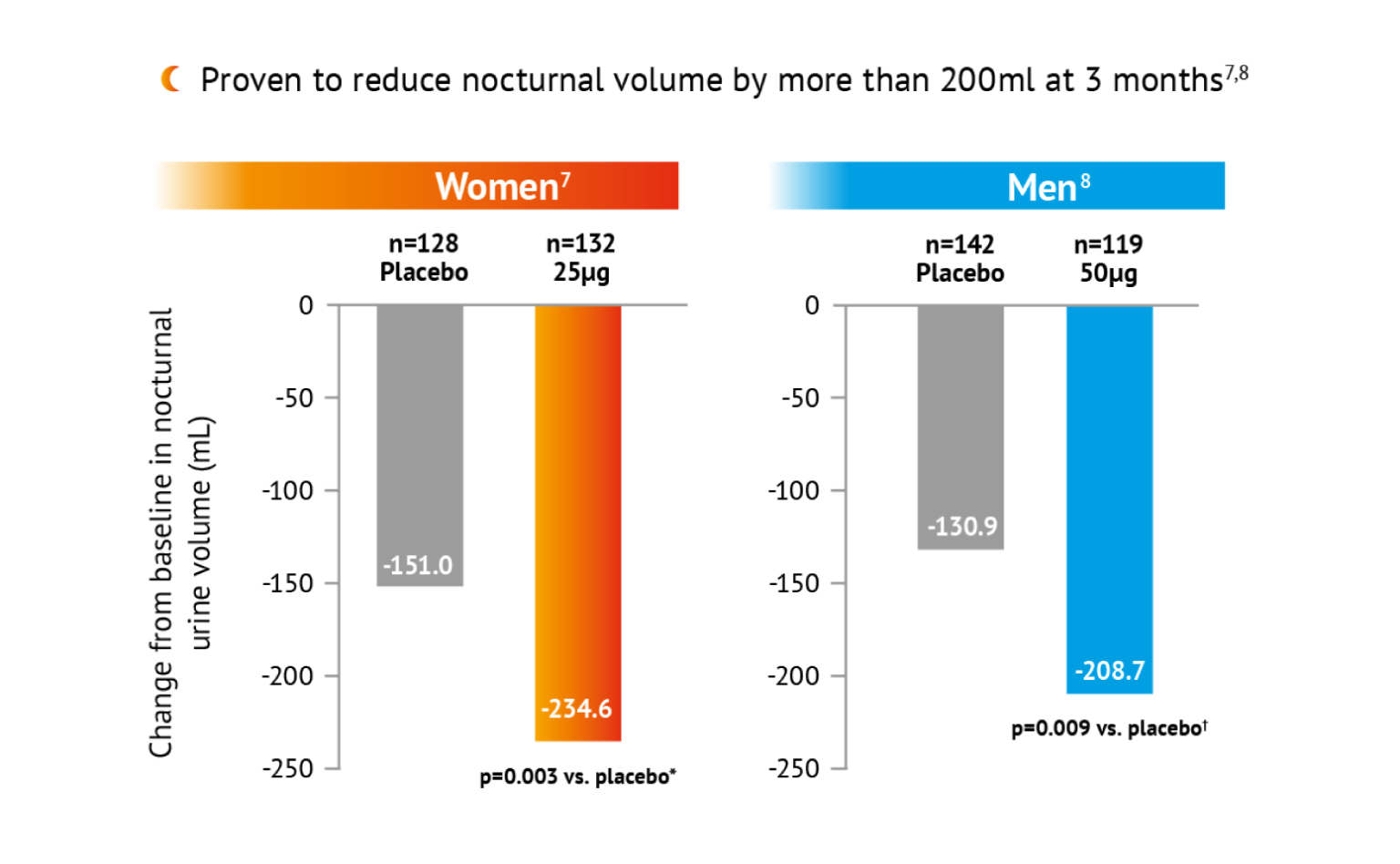
*Treatment contrast / odds ratio -83.56 (95% CI: -138.74, -28.38). †Treatment contrast/odds ratio -83.56 (95% Cl:5.7, -19.9). Placebo = Standard of care Including lifestyle modifications
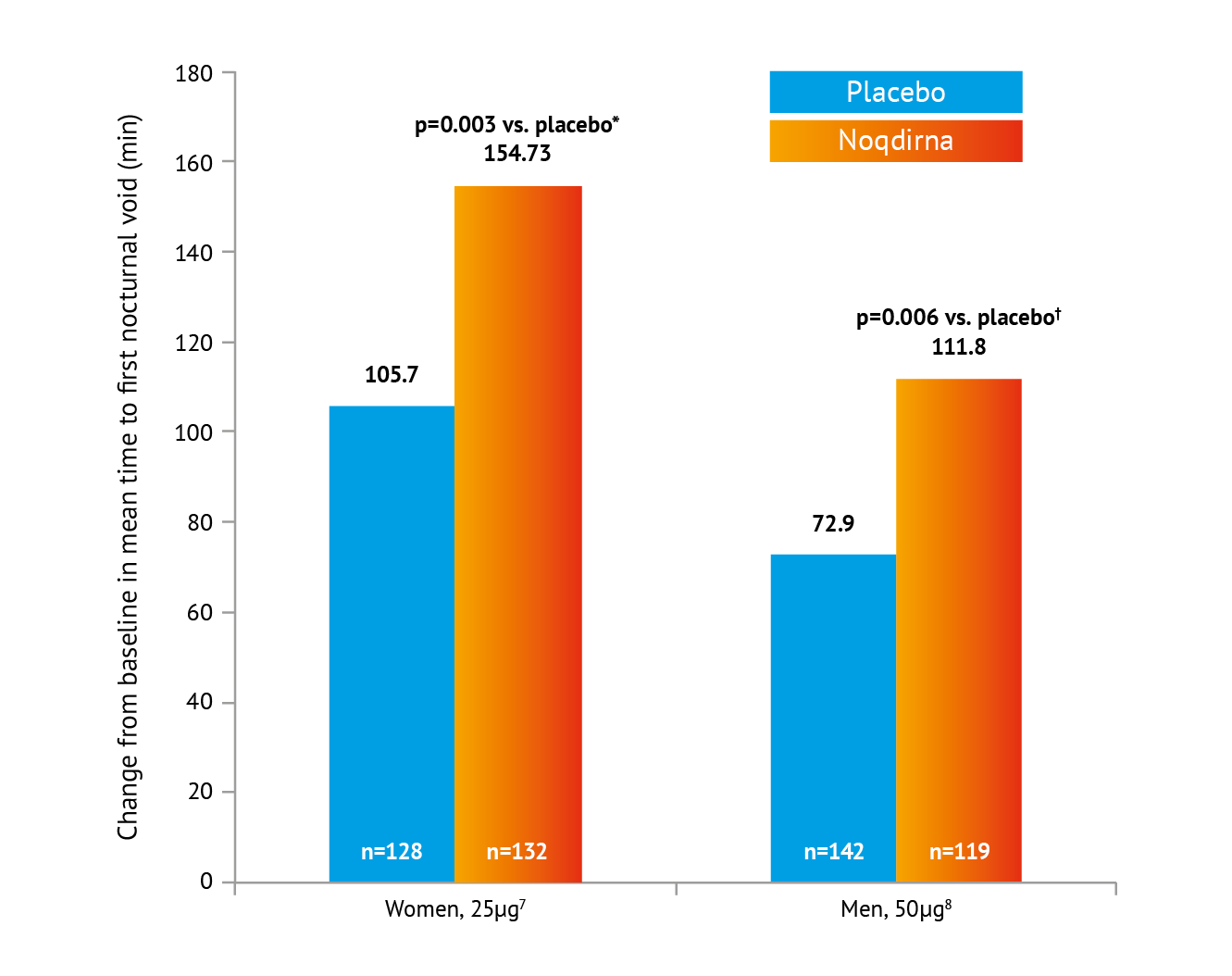
*Treatment contrast / odds ratio 49.03 (95% CI: 16.35, 81.70). †Treatment contrast / odds ratio 39.0 (es% Cl: 11.0, 66.9). Placebo = Standard of care Including lifestyle modifications
Noqdirna demonstrated the ability to increase time to first void by an average of 39-49 minutes at month 3 vs placebo,7,8 further resulting in a mean total initial undisturbed sleep period of approximately 4.5 hours at 3 months in one treatment group, significantly improving patient quality of life.8
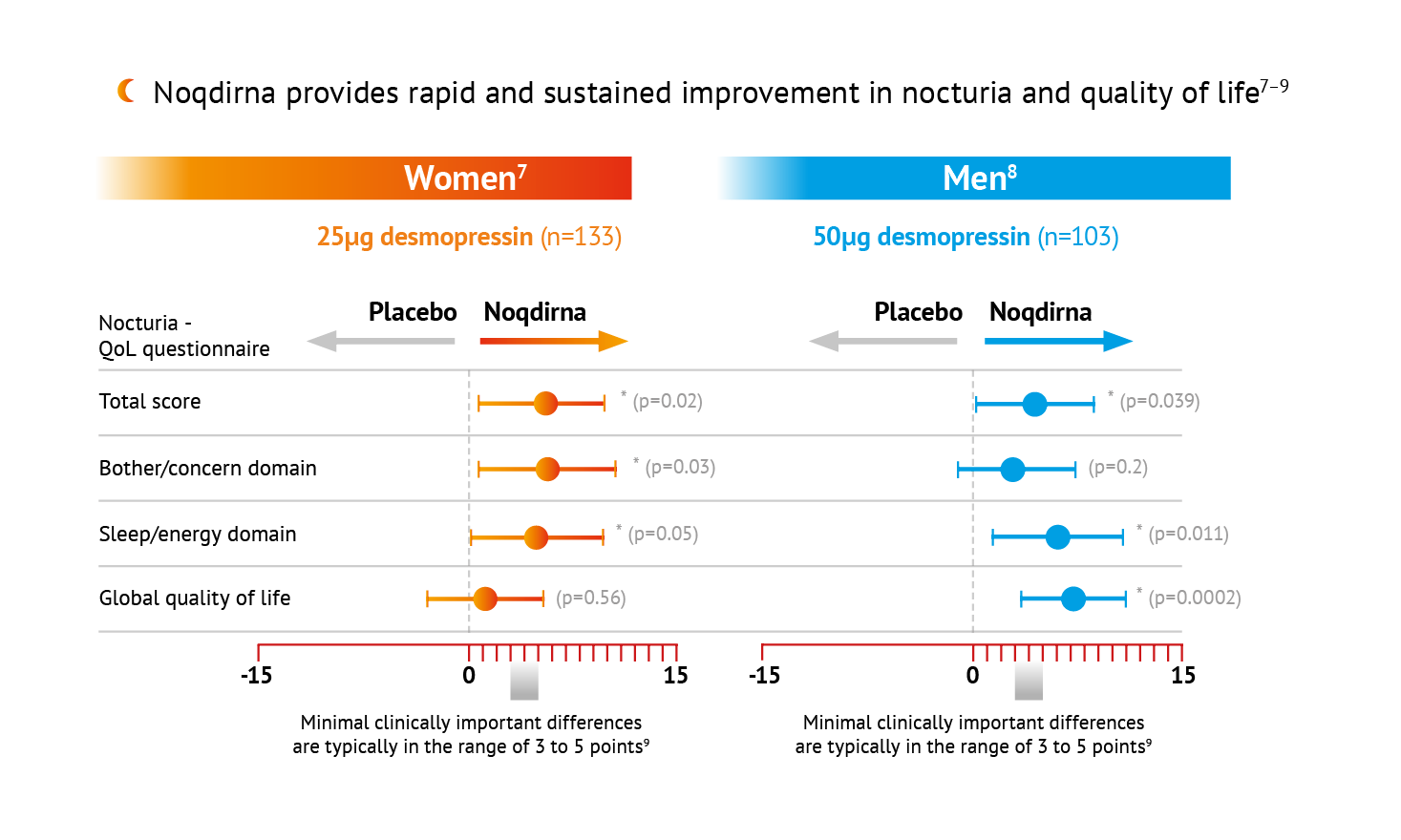
Statistically significant difference vs. placebo, repeated measures ANCOVA ps0.05
The use of Noqdirna in patients with nocturnal polyuria has been shown to be well-tolerated in both male and female patients.7,8 Patient-reported outcomes demonstrated statistically significant reduction (p=0.03) in ctivity impairment compared to the placebo group.7 *Noqdirna, a low-dose desmopressin with gender-based dosing, reduces the risk of serious adverse events such as hyponatraemia.1 Click here to access a guide to managing your patients with idiopathic nocturnal polyuria and hyponatraemia.

*Noqdirna contraindications include hypersensitivity of the active substances or to any of the excipients, habitual or psychogenic polydipsia (resulting in a urine production exceeding 40 mVkg/24 hours), known or suspected cardiac insufficiency or other conditions associated with fluid overload, sufficient to require treatment with diuretics (including history of such conditions), moderate and severe renal insufficiency (creatinine clearance below 50 ml/min), known history of hyponatraemia and syndrome of inappropriate antidiuretic hormone (ADH) secretion (SIADH).1
Job Code: UK-NOQD-2000015 - Date of preparation: January 2023