


Adverse Event reporting information can be found in footer
Request a Meeting
(desmopressin [as acetate] oral lyophilisate)
DesmoMelt is well accepted by children of various ages and facilitates early intervention2, providing an effective and convenient solution to primary nocturnal enuresis and helping children and families return to a normal life.
Due to the superior pharmacodynamic characteristics of the melt formulation, more than 25% of patients had a higher diuresis rate with the tablet vs. melt formulation, which was significantly different from hours 3 to 8 after dosing3.

DesmoMelt is associated with a numerically higher compliance rate than the tablet (94.5% vs. 88.9%; P=0.059, not significant) and retains similar levels of efficacy and safety at lower dosing levels2.
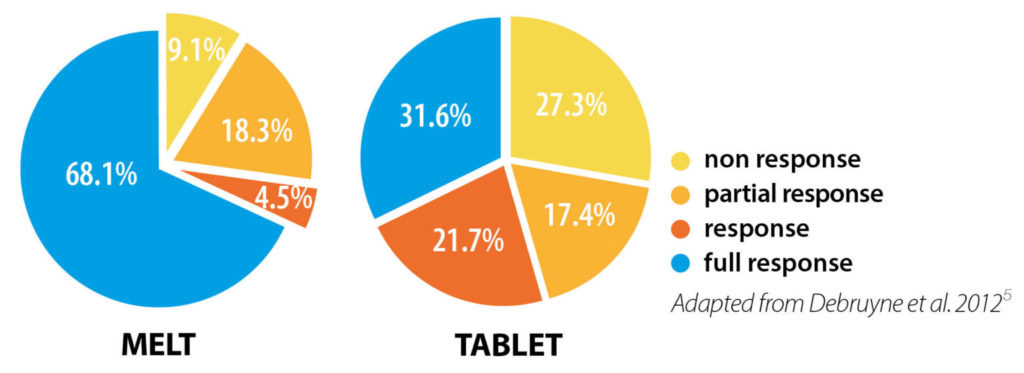
In a study comparing children’s response rates after 2 months’ treatment, overall response rates were better with DesmoMelt5
A single dose (120mcg) of DesmoMelt should be taken before bedtime for a period of 3 months, followed by a 1 week drug-free period to assess results. If symptoms persist, the treatment cycle should be repeated1.
If needed, the dose taken can be increased to 240mcg1.

DesmoMelt is intended for treatment periods of up to 3 months. The need for continued treatment should be reassessed by means of a period of at least 1 week without DesmoMelt1.
For more information about the benefits of DesmoMelt,
Job Code: UK-MN-2200029 - Date of preparation: March 2023